For decades rehabilitation from SCI has focused on compensating for what has been lost through injury rather than on recovery. Research performed in recent years has discovered that with appropriately designed exercises, the spinal cord can in some cases be retrained to perform lost functions. The ability of a nervous system to adapt to performing tasks after injury is known as “plasticity.” For example, if a part of the brain is injured that is normally solely responsible for performing a certain function, other parts of the brain that are not injured may learn to perform that function. We now know that the spinal cord has a certain amount of plasticity. Laboratory animals with complete severing of the spinal cord have been able to relearn some lost functions. For example, a rat has a very awkward, slow, wide based gait in its hind limbs following complete severing of the spinal cord in the middle of its back. However, these rats can be taught to step at a much faster pace. The retraining requires weight-bearing on the hind limbs and stepping on a treadmill. This is accomplished with robotic devices that apply force to the limbs while also coordinating the non-weight bearing “swing” phase of the limbs while walking. This ability of the spinal cord to learn indicates that when input from the brain is lost, groups of spinal cord neurons can learn to coordinate the actions of the muscles involved in walking. Beginning research involving people with thoracic SCIs has found that the spinal cord can be retrained in some people to help with walking (people with incomplete SCI injuries tend to perform better). Robotic equipment is now being developed to help with the training process, both to make the training very precise and to adapt the training program as relearning occurs.
Research and development are quickly proceeding on electrical devices (neural prostheses) to restore functions lost following SCI, including the ability to control urination, standing/walking, and hand grasp. The VOCARE bladder system, which until recently was marketed by NeuroControl Corporation, has been implanted in 2,000 SCI survivors, most of whom live in Europe. The device is being clinically tested in the United States.
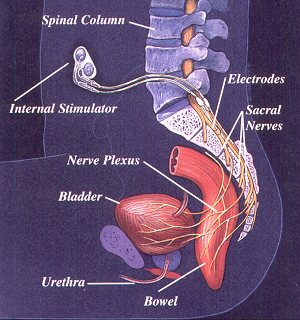
The bladder system has an “internal stimulator” that is surgically implanted under the skin in the chest or abdomen. It is connected to electrodes that are implanted in the spine in the area where nerves from the bladder and bowel connect. The device is operated by an external controller. When the controller is turned on it sends a signal to the internal stimulator, causing the bladder to contract and empty. It can also help with emptying of the bowel and allows some men with the implant to have an erection.
Neopraxis, Ltd., an Australian company, has developed a system to enable SCI survivors with thoracic injuries to stand and walk (for short distances). It consists of an external controller worn on the body, a transmitting coil, an implanted 22-channel stimulator, and surgically implanted electrodes in the legs, hip region, gluteal muscles, and sacrum. External sensor packs, each containing a gyroscope and two accelerometers, are attached to the thigh, calf, and trunk to provide real-time feedback on body position. The system is undergoing investigational trials in the U.S. SCI survivors train with the device using an external stimulator for several weeks before an internal stimulator is implanted.
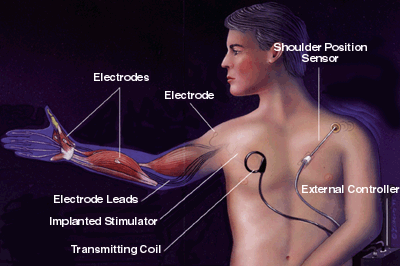
Two devices have been developed for SCI survivors with cervical injuries to allow them to grasp objects. One is called the Freehand system, which requires surgical implantation of electrodes and a stimulator. The device requires that the survivor still have some control of upper shoulder and neck muscles. Users undergo an average of three months of training to learn how to control their hand movements with small shrugs of the opposite shoulder. One shrug sends a signal telling the hand muscles to pinch thumb and finger together. Another shrug tells the hand to lock in place. The system allows some quadriplegics to independently feed themselves and do other simple tasks with a hand.
The other device, called the Handmaster, contains five external electrodes in a splint worn on the arm. No surgery is required. It has an LCD screen on the controller and enables the user to extend and flex thumb and forefinger. There are seven different modes of electrical stimulation that the user can choose.
One of the challenges with the neural prostheses is that they are currently very expensive. For example, when the Handmaster system was first marketed it cost $50,000. The market for products like the Handmaster is also small. Most SCI survivors are paraplegics and not quadriplegics. And while there are approximately 10,000 people in the United States who suffer a serious SCI every year, this is a small fraction of the number of people affected by conditions such as strokes. Consequently, there have been very few companies that have brought neural prostheses for SCI survivors to market and some of those companies have either ceased operations or quit marketing products for SCI. Fortunately, research on the products is continuing at universities and grants are being made available in both Europe and the United States for prosthetic design. In the United States alone, there are more than a dozen spinal cord injury research centers at universities.
Another research goal is to minimize the damage to the spinal cord in patients who have recently suffered an SCI. Only part of the damage to the spinal cord occurs at the moment of trauma. Additional damage (called secondary damage) occurs in the hours and days following the initial injury. The secondary damage is caused by the body’s response to the trauma. Delayed injury presents a window of opportunity for treatment aimed at reducing secondary damage.
Most types of cells that are part of our immune systems enter the spinal cord only rarely. However, when the spinal cord is damaged by trauma, immune cells invade, removing dead cells and releasing powerful chemicals, both helpful and harmful. In the harmful category are highly reactive chemicals called oxidants or "free radicals." These chemicals attack the body's natural defenses and damage spinal cord cells.
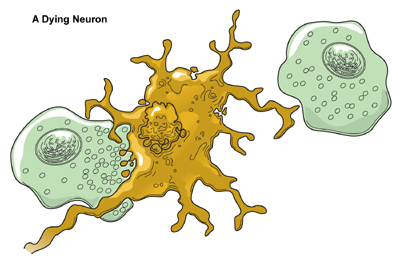
Macrophages (one type of immune cell which is green in the diagram) eat dying neurons in order to clear debris.
Trauma also causes nerve cells to become “overexcited,” leading to further damage. Gaining a better understanding of how to block these processes from occurring will provide new treatments to reduce damage following spinal cord injury. One treatment already in use is methylprednisolone, a steroid medication that has somewhat improved the outcome for patients with SCIs if administration is begun within eight hours of the injury.
Until recently most cell death in spinal cord injury was attributed to the common, uncontrolled form of cell death in which cells swell and break open. This common process is called necrosis. Recent experiments have shown that some cells die as a result of a different process called apoptosis, a form of "cell suicide" in which damaged cells eliminate themselves in a more controlled way than simply swelling and bursting. Controlling apoptosis appears to improve recovery after spinal cord injury in rodents.
Damage to axons causes most of the problems associated with spinal cord injury. Until recently, most researchers assumed that trauma damaged axons at the time of injury by stretching or tearing them. New evidence suggests that much of the injury to axons is not immediate but instead results gradually from disruption in the axon’s ability to transport materials needed for survival from one part of the axon to another. This delay in axon loss allows time for intervention. One such promising intervention has been successfully used by veterinarians treating dogs who have been paralyzed as a result of a ruptured disc. The dogs are given an intravenous drug (a hydrophilic polymer) that is known to help cells repair the membranes that surround and protect them. The treated dogs have had a much better recovery than dogs not given the treatment.
The central nervous system is an incredibly complex organ. That complexity enables us to do wonderfully complex tasks, but it also makes finding a cure for serious injuries of the spinal cord a very difficult task. However, research on understanding how the spinal cord develops and functions is proceeding rapidly, leading to a growing hope among researchers that repairs of seriously injured spinal cords will be possible in the foreseeable future. In the meantime, better rehabilitation, neural prostheses, and reduction of secondary damage to the spinal cord offer hope that SCI survivors can have improved functioning while waiting for a cure.
Stem Cell Research into SCI Therapy
Stem cell research in pursuit of spinal cord injury treatments
Pain management for SCI patients
Research studies on experimental spinal cord injury treatments need volunteers.