Teriparatide is a recombinant segment of human parathyroid hormone, containing its first 34 amino acids. You might see if referred to as PTH 1-34. It is manufactured via biotechnology, using the microorganism escherichia coli. When parathyroid hormone is constantly present it can actually cause bone loss. But when teriparatide is given in a pulse, once a day, it promotes new bone formation.
The drug is injected into the subcutaneous tissue. Peak concentration in the blood occurs quickly and the half-life i 75 minutes. So the body and bones experience the hormone as a pulse-like assault, which results in bone mineralization.
Other osteoporosis therapies act mainly to inhibit bone resorption and reduce bone remodeling. These are called "antiresorptive agents". Teriparatide is actually an osteoanabolic drug - it acts to build up bones and has the potential to improve skeletal microarchitecture and volume.
Osteoblasts are the cells that lay down mineral in the bone. Teriparatide promotes the production of osteoblasts from progenitor cells and slows the death of existing osteoblasts. How? It appears to trigger several growth factors in the bone tissue, including production of insulin-like growth factor-1.
Parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP) are true osteoanabolics. Giving patients parathyroid hormone-related protein (PTHrP)(1-36) also results in an increase in bone mass. Inside the body this protein promotes the release of parathyroid hormone. It has sparked some interest among researchers, but no therapies have been developed, partly due to concerns the protein will increase the risk of hypercalcemia.
There were two small studies comparing combination therapy, using teriparatide with alendronate, one study with men, one with women. Both showed more increased bone mineral density in the spine with the combination than with alendronate alone, but not more than with teriparatide alone. Another study by biomechanical engnieers found a no difference in femoral strength in patients treated with teriparatide versus patients treated with alendronate.
Some recent research has tied a PTH molecule to a bifunctional biphosphonate for delivery to the bone tissue. This isn't used clinically, but it is an exciting development.
Teriparatide has also been shown effective in treating women with anorexia nervose.
The FDA has not approved teriparatide for treatment of fractures, but doctors have employed it “off-label” for that purpose. Formal studies indicate it is a good treatment. The FDA label for teriparatide says patients should spend no more than two years on the drug. However, now that we have a good deal of experience with the drug and there is no indication of increases of osteosarcoma rates, so many observers hope the FDA will change the label.
Researchers have modified parathyroid hormone so that it doesn’t break down as fast, at least in laboratory animals. Peptides break down in the body, and by modifying the “backbone” of the molecule so that concentrations remain higher at the cellular level over a period of time the effectiveness of the drug may increase. Down the line, this may allow development of a drug that can be swallowed in pill form.
Teriparatide has the disadvantage of needing to be administered by injection daily, something that many people are not comfortable doing. The recommended dose is 20 micrograms daily, given into the thigh or abdomen. It comes in a preloaded pen with 28 doses. A doctor, nurse, or pharmacist should explain how to use it. It should be kept in the refrigerator. A new needle must be used for each injection, and the remaining medicine returned to the refrigerator. The pharmacist should also explain a way to safely dispose of needles.
The reason the drug must be taken every day is because the body eliminates it so quickly. The concentration in the bloodstream peaks about 30 minutes after injection. The half-life is abour one hour when administered by subcutaneous injection. Injecting the drug makes the bioavailability 95%, which is much higher than the bioavailability of drugs that are swallowed.
Within three hours, the drug is down to such low levels that it is essentially gone. The body clears the drug as it normally clears external materials.
Engineers have recently devised a way to put teriparatide in a mechanical device "about the size of a domino" which is implanted in the body and releases the drug over time. If this proves feasible, it would reduce the need for patients to do daily injections. Most likely it will take years for this product to become available even it is is feasible.
Duration of treatment is 18-24 months. Markers showing progress against osteoporosis peak after 6-12 months. Doctors evaluate progress during this period and may recommend continuing treatment.
The goals of osteoporosis treatment are generally to stop or slow bone density decline, to prevent fractures, and to control pain from the disease. Combination therapy of teriparatide plus another agent appears to work well.
New microneedle technology may be feasible for teriparatide administration in the future.
Regimens of teriparatide plus zolendronic acid or alendronate show better improvement in bone density that when only one agent is given. Women on hormone replacement therapy have better inceases in bone strenth. There are risks and other considerations before going with a combination therapy, so ask your doctor.
A 2006 study published in the Journal of Drug Assessment concluded that although teriparatide is the most expensive therapy for preventing bone fractures in osteoporosis patients, it is on the "cost-effectiveness frontier" - meaning it is a good choice for some patients.
Recommendations released by the American College of Physicians in September 2008 included the statement that doctors should offer drugs to people with osteoporosis, and that doctors should consider preventative treatment for osteoporosis. The organization said teriparatide prevented spine fractures, but noted that evidence for other types of fractures is mixed.
In Dec 2011 the pharmaceutical firm Unigene announced it was working on an oral form of recombinant parathyroid hormone for osteoporosis treatment. GlaxoSmith Kline had the option to pick up development of Unigene's product, but chose not to. This drug is currently in Phase II trials.
Many long-term intractable diseases are treated with combinations of drugs. Cancer is often addressed this way. The idea is that if you attack the disease through more than one biochemical pathway. Teriparatide plus bisphosphonates (Fosamax, Boniva, etc.) is one possible combination. A small clinical trial has demonstrated efficacy in increasing bone density with a combination of denosumab and teriparatide. The increase was found to be more than would be expected by each medicine by itself.
It turns out the teriparatide followed by a bisphosphonate helps maintain the gains in bone. When teriparatide therapy is ended, if no antiresoprtive therapy is applied, the bone density declines. This has been shown in more than one study. Most teriparatide patients have been given a bisphosphonate for some period before their teriparatide regimen - often for years - because bisphosphonate therapy is the first-line in osteoporosis treatment.
Researchers did a test where teriparatide was given again to people who had already been through one course of treatment. The first course lasted 30 months and then patients went drug-free for a period followed by a second course. During the interim drug-free period bone density declines, but researchers were wondering whether the second course would result in as rapid improvements as the first course did. Unfortunately, retreatment was not as effective. On average bone density increased 5.2% in the first 12 months of the second course, compared to 12.5% in the first 12 months of the first course.
Scientists tested teriparatide in animals and found it could halt loss of cartilage in injured joints and even cause cartilage to grow in those joints. This gives hope that the drug may find use in treatment of osteoarthritis. In arthritis the synovial membrane is inflamed and there is destruction of bone and cartilage. Eli Lilly is currently running a clinical trial to get more data on this question.
FLEX Study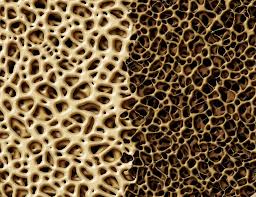
Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective. More
Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. More.
