Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. They can have different effects on different tissues so they may block estrogen’s action in breast cells and activate estrogen’s action in other cells such as uterine or bone cells. Physiologically SERMs can act as either estrogen agonists or estrogen antagonists. As drugs, SERMs are used to treat cancer, osteoporosis, menopause symptoms and halted ovulation. They can prevent the growth of estrogen-sensitive cancer cells by taking the place of the estrogen in the receptors so the estrogen cannot enter.
In bone, estrogen receptors ERa and ERß are present in the cells - osteoblastes, osteocytes, and condrocytes - of the growth plate cartilage.
The first SERM in wide clinical use was tamoxifen. It was originally developed as a contraceptive because of its anti-estrogen effects, but proved not useful for birth control. In 1977 the Federal Drug Administration (FDA) approved its use for advanced breast cancer, because in tests and clinical trials it proved to deprive the cancer cells of estrogen. It was also successful in prolonging the survival rate for women who had had breast cancer surgery as well as lymph cancer that was less advanced. Tamoxifen was approved for both breast cancer surgery adjuvant chemotherapy and for lymph cancer in 1980.
The major SERM used for osteoporosis, raloxifene, was approved by the FDA in 2007 for women with osteoporosis or considered to have a good possibility for invasive breast cancer. The FDA approval was based on tests of almost 20,000 women to measure the difference between raloxifene and tamoxifen in reducing the probability of breast cancer. Raloxifene reduced by 50% the incidence of invasive breast cancer compared to tamoxifen, blood clots by 29% and cancer of the uterus by 36%. Raloxifene (sold under the name Evista) has been shown to reduce bone turnover in postmenopausal women. Its activity in fighting both breast cancer and osteoporosis make it an attractive drug.
Raloxifene is currently the main SERM used for osteoporosis. Bazedoxifene is used as part of a combination medicine.
Arzoxifene is SERM which has anti-estrogen activity. Eli Lilly developed it as an osteoporosis treatment, but clinical trials showed poor results - it did not reduce nonspinal fractures and it increased the risk of blood clots - and Lilly dropped development efforts on arzoxifene. Toremifene is another estrogen blocker that started being used in 2008 for metastatic breast cancer and is being considered for prostate cancer prevention.
Bazedoxifene (http://www.ncbi.nlm.nih.gov/pubmed/21187592) has been approved for osteoporosis treatment in Europe and Japan, and as part of a combination therapy in the United States. The combination therapy is called Duavee and mixed bazedoxifene in the same pill with conjugated estrogens. It has been shown to reduce incidence of fractures in osteoporosis patients.
Lasofoxifene is also used in Europe by not approved in the US and its maker, Pfizer, does not appear to be pushing for approval any more. Tests had shown it was as effective as raloxifene in firming up bones as well as reducing the risk of breat cancer and vaginal atrophy.
Femarelle (scientific name DT56a) is a dietary supplement sold in Europe but has never been approved by the US Food and Drug Administration. It is advertised mostly for relief of menopause symptoms.
What is the benefit of having more than one SERM available, especially after Evista goes off-patent and becomes available as an inexpensive generic? It gets back to personalized treatment. Different physiologies benefit more or less from different medicines. The "ideal SERM" - which helps increase bone density while having antiestrogenic activity in breast tisse and little or no impact on uterine tissue - may not exist for the general population. But particular SERMs may be close to ideal for particular groups of patients.
The SERMs Ospemifene and Lasofoxifene can be used to treat vaginal atrophy.
The possible side effects of SERMs include blood clots, endometrial cancer and stroke. If there is any history of stroke or blood clots or if the patient smokes, the doctor should be informed immediately. The side effects most often seen are hot flashes, vaginal discharge, fatigue, mood swings, low libido and night sweats, but there are other less common side effects including endometrial thickening (or hyperplasia), risk of uterine cancer, dizziness, severe headaches and chest pain.
There have also been several studies comparing raloxifene to alendronate . The bisphosphonate alendronate increased bone density more than raloxifene did. Results were inconclusive as to whether alendronate or raloxifene was better for reducing the risk of fractures.
Related: Selective Androgen Receptor Modulators
Journal abstracts: SERMs in the prevention and treatment of postmenopausal osteoporosis
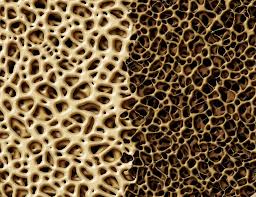
Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective. More
Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. More.
