Gastroesophageal reflux disease (GERD) or heartburn is a common malady. The acid in the stomach irritates tissue where there isn’t enough protective lining. If the acid gets outside the stomach, the patient feels pain or unease. Modern prescription drugs such as Nexium and Prevacid - classified as proton-pump inhibitors or H2 inhibitors - work by interrupting the physiological mechanism of acid production and distribution, but many people use over-the-counter medicines for heartburn. The OTC drugs are called antacids.
Antacids counteract acid by neutralizing it in a chemical sense. The pills are alkaline (aka bases) and the method of action is simple. Some antacids are prescription medications, but many are available over the counter and modern drug stores dedicate serious shelf space to these products. The antacids are almost always compounds of magnesium, calcium, or aluminum.
The obvious short-term side effects are in the digestive system, but in the long run with extended use they can have an effect on the skeletal system. Aluminum-containing compounds in particular have been known to result in detrimental changes to the bone as the aluminum displaces some of the body's calcium. These are found in Maalox, Mylanta, Riopan. Calcium-containing compounds, on the other hand, can have unintended good benefits as they essentially act as a dietary calcium supplement.
During the 1980s Tums, which consists of calcium compounds. aired television commercials touting this added benefit and reminding viewers that your body needs calcium anyway, so you might as well choose Tums.
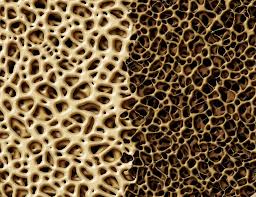
The concern is that antacid use might result in decreasing calcium levels in the skeletal system. Less calcium means less dense bones, and subsequent increase in risk of bone fracture. Indeed, it has been found that a density that is one standard deviation below (at any place in the skeleton) the baseline level for young adults results in an increase of hip fracture risk by almost 100 percent.
Skip the Calcium?
Antacids that produce aluminum hydroxide or magnesium hydroxide in the body; these materials can bind with phosphate ions in the digestive system, essentially reducing the amount of phosphorus entering the body. In short term use this is not normally a problem, but chronic use may result in depletion of phosphorus in the body and softening of bones. Cases of osteopenia have been caused by excessive consumption of aluminum and magnesium compounds. Weak bones are typically caused by not enough calcium - calcium is the limiting nutrient and there is plenty of phosphorus. This is a situation where the bone softening was due to a lack of phosphorus.
There is some evidence of depletion of other trace minerals due to antacid use; these include zinc, copper, potassium, and iron.
Bisphosphonates block bone resorption. They have been widely used for years and although there can be side effects, the medical profession considers them generally safe and effective. More
Selective Estrogen Receptor Modulators (SERMs) are chemical compounds that act on the estrogen receptors in the body. More.
